Aster Spathulifolius
What is Aster Spathulifolius?

Aster Spathulifolius
Aster spathulifolius is a dicotyledonous plant in herbaceous perennial campanulales found at the seaside.
Aster spathulifolius
- It grows obliquely up to 30-60 cm. The leaves grow jig jag in a shape of upside-down egg and concentrated on the lower part of the plant. The both sides of the leaves are covered with hair. They look pale and their edges are smooth or slightly jagged.
- The flowers, light purple in color, bloom from July to November. The fruit matures in November. The pappus is light brown and covered with tough hair. Its leaves are edible.
- Aster spathulifolius is found in the south of the central Korea and Japan. The main producing areas in Korea are Ulleungdo Island, Gyeonggi-do, and Jeju Island. There is no known method for cultivation. The plant grows in the wild and harvested between June and July, as well as October and November.
- Traditionally, the young leaves of the plant can be eaten, and the whole plant is used to treat diabetes, bladder infections, and other diseases. The plant is also grown for ornamental purposes due to its ability to withstand cold winters and its lignification properties. The blood lipid reduction effect and antiobesity effect of the above-ground part of Aster spathulifolius were proven by a research team of Kyung Hee University.


The standardization of Aster spathulifolius extract
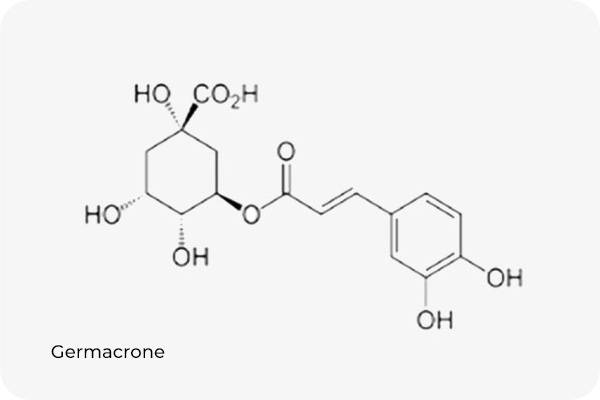
The marker compound of Aster spathulifolius is germacrone. Aster spathulifolius extract’s germacrone content is standardized to be at least 1%.
The safety of Aster spathulifolius extract
No Mutagenicity or Genotoxicity.
- Chromosomal Aberration Test
: Negative test result - Mouse lymphoma test
: Negative - Micronucleus Test
: Negative test result
Human Clinical Studies
No side effects reported
Listed as an edible ingredient in the Ministry of Food and Drug Safety's food ingredient database
Acute oral toxicity test
Injecting more than 5,000 mg/kg b.w. of LD50 to male and female mice
13-week repeat dose test
The NOAEL value was 2,000mg/kg/day on both females and males
The efficacy of Aster spathulifolius
-
Body fat reduction
The body weight, body fat, waist-hip proportion, visceral fat, and subcutaneous fat are significantly reduced in a human clinical trial.

-
Blood sugar improvement
ood sugar level control and insulin resistance were significantly increased in an animal test.
Papers related to Aster spathulifolius extract
- In vitro efficacy evaluation for preventing diabetes and diabetic complications from Aster spathulifolius (Food science and biotechnology, 2015, 24(1), 301-306)
- Inhibitory effects of Aster spathulifolius extract on adipogenesis and lipid accumulation in 3T3-L1 preadipocytes
(Journal of Pharmacy and Pharmacology; in press) - Anti-Diabetic Effect of Aster sphathulifolius in C57BL/KsJ-db/db Mice(Journal of Medicinal Food; in press)
- Anti-obesity effects of Aster spathulifolius extract in high-fat diet-induced obese rats
(Journal of Medicinal Food; in press)
Clinical Study : Weight Management
- – Test substance: Aster spathulifolius extract 700mg/day
- – Test design: Randomized, double-blind, placebo-controlled, 12 weeks
- – Test subjects: n=40, average body weight 77kg, 25-30 BMI (kg/㎡_
- – Analysis method: Intention To Treat (ITT) analysis/ANCOVA
Body weight & Body fat mass reduction
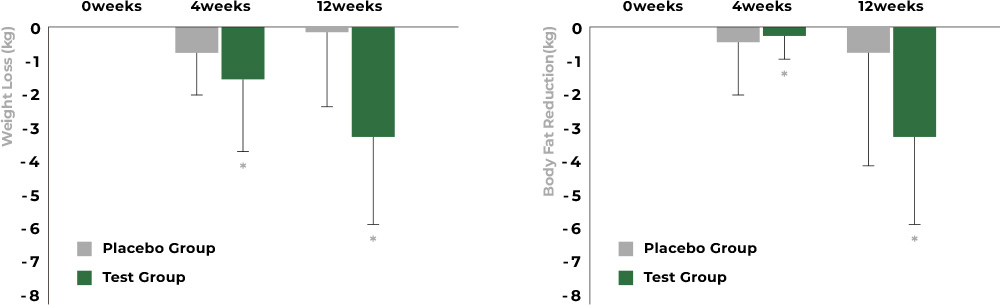
When comparing body weight and BMI at each ingestion period, the group that consumed Aster spathulifolius extract showed significant reductions in both body weight and body fat.
(At 12 ingestions, body weight is reduced by 3.3kg (p=0.0004), body fat is reduced by 2.38kg (p=0.0075))
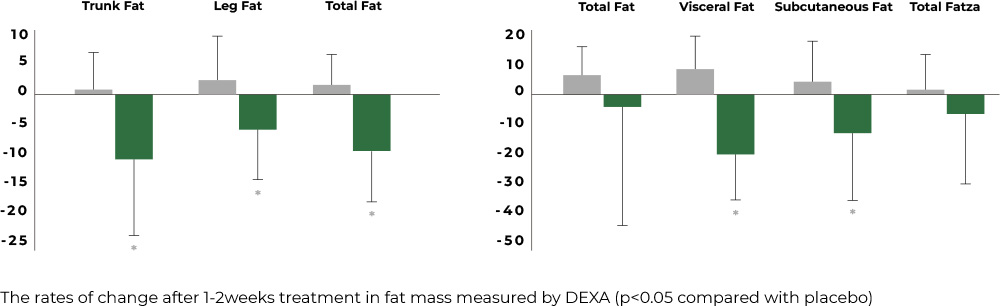
Reduction of body fat (total fat, trunk fat, leg fat), visceral fat, and subcutaneous fat
Twelve weeks after the ingestion of Aster spathulifolius extract, the group that consumed the extract showed significant reductions in body fat (including total fat, trunk fat, and leg fat), visceral fat, and subcutaneous fat when compared to their initial measurements.
The body weight control mechanism of Aster spathulifolius extract
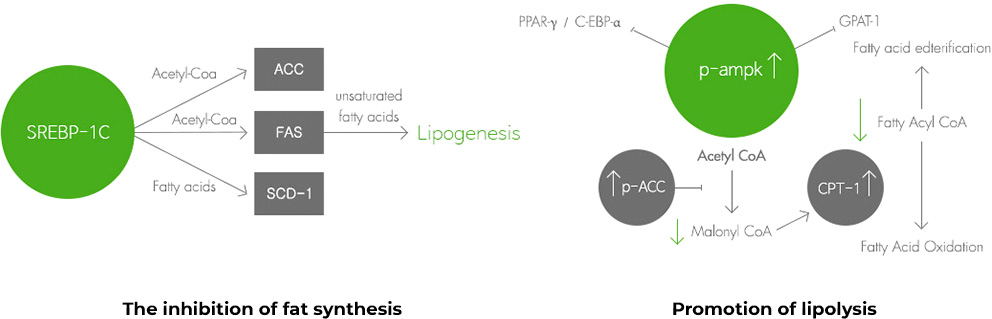

Animal Study of Weight Management
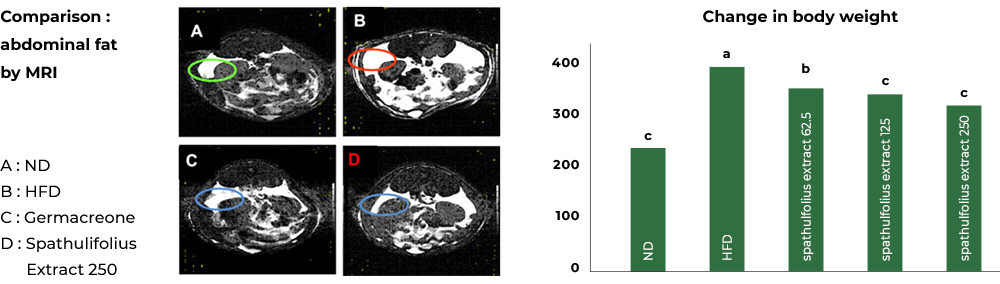
1st study : Reduced abdominal fat and body weight
In the study involving mice fed a high-fat diet, the group fed a high-fat diet together with Aster spathulifolius extract for 12 weeks had their abdomen fat reduced and the body weight reduced significantly when compared to the group with a high-fat diet only.
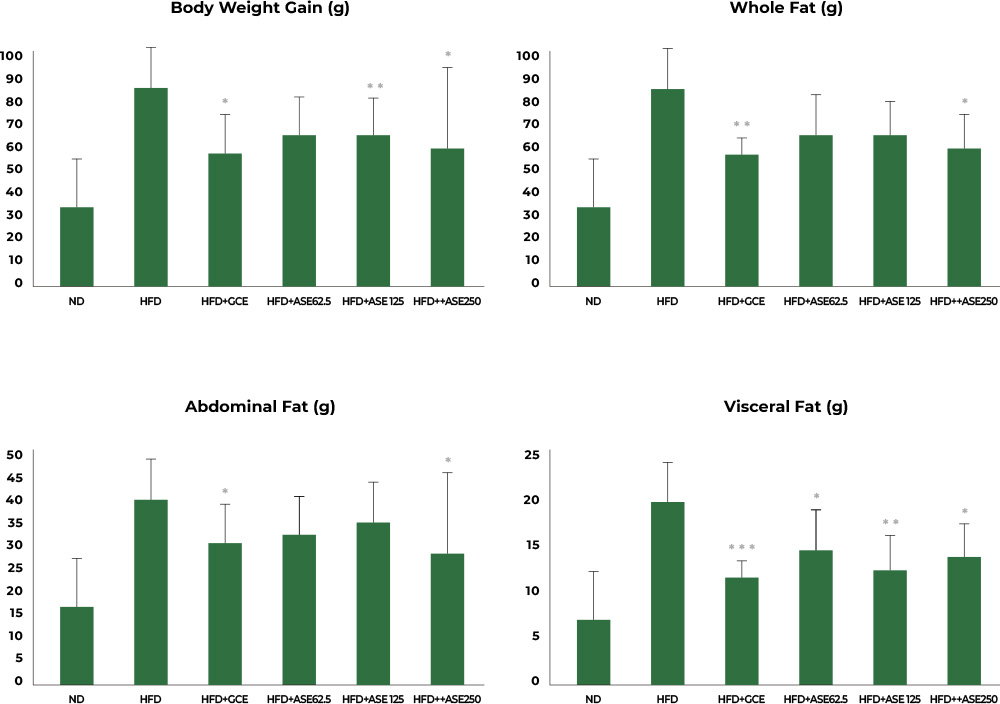
2nd study : Reduced body fat and visceral fat
In the study involving mice fed a high-fat diet, the group fed a high-fat diet together with Aster spathulifolius extract for 12 weeks had their body weight, body fat, visceral fat, and subcutaneous fat reduced significantly when compared to the group with a high-fat diet only.
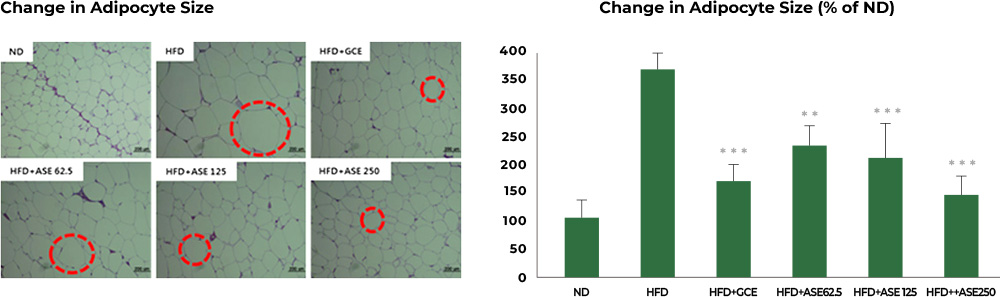
2nd study : Reduced fat cell size
In the study involving mice fed a high-fat diet, the group fed a high-fat diet together with Aster spathulifolius extract for 12 weeks had their fat cell size reduced significantly when compared to the group with a high-fat diet only.
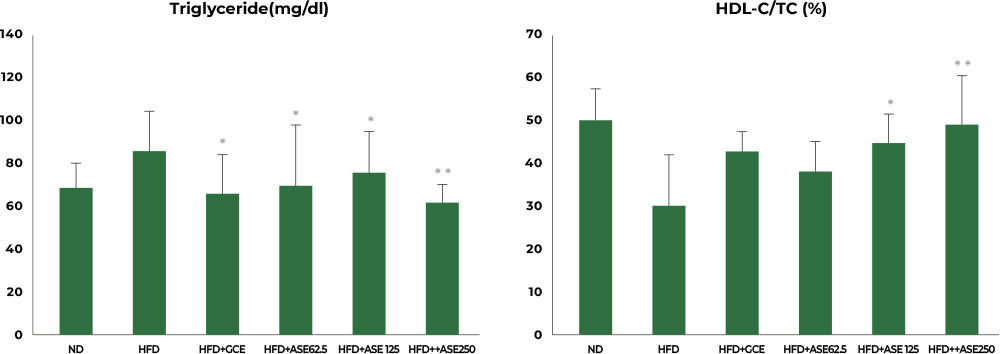
2nd study : Reduced TG and TC, increased HDL-C/TC
In the study involving mice fed a high-fat diet, the group fed a high-fat diet together with Aster spathulifolius extract for 12 weeks had their triglyceride, total cholesterol, and HDL-C/TC significantly improved, corresponding to concentration level, when compared to the group with a high-fat diet only.
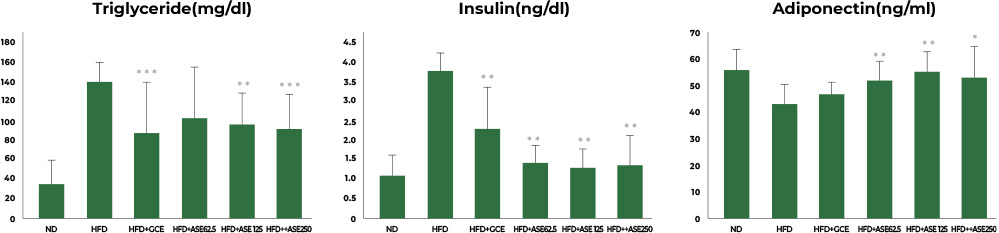
2nd study : Reduced leptin, insulin, and adiponectin
In the study involving mice fed a high-fat diet, the group fed a high-fat diet together with Aster spathulifolius extract for 12 weeks had their leptin, insulin, and adiponectin significantly improved, corresponding to concentration level, when compared to the group with a high-fat diet only.
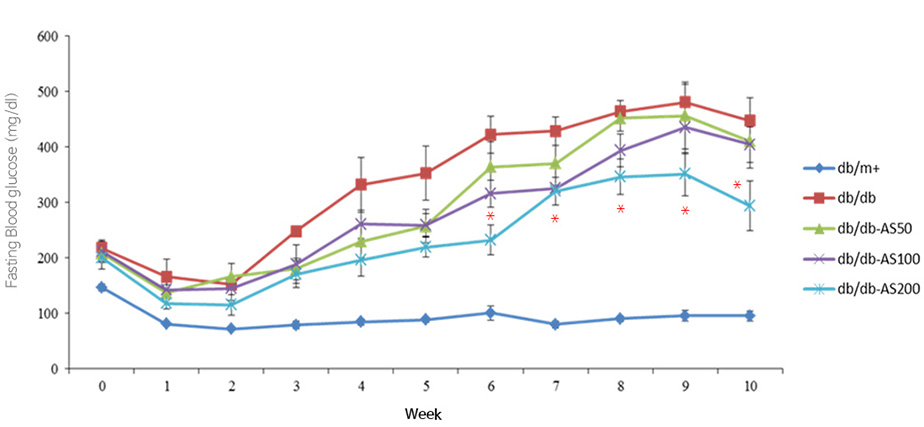
Animal study of Blood glucose and insulin resistance
Reduced fasting blood sugar
- Weekly changes in the fasting blood glucose levels of C57BL/KsJ db/db mice that were administered Aster sphathulifolius extract
- A significant blood sugar level reduction was observed from the group that consumed Aster spathulifolius extract 200mg/kg/day by the sixth week
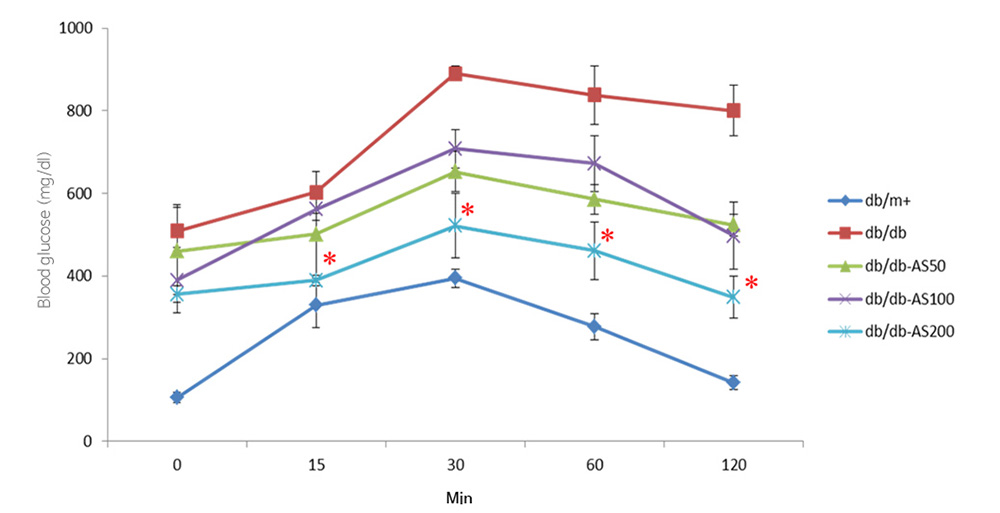
Intraperitoneal glucose tolerance test (IPGTT)
It is determined that the extract improves the insulin resistance of type 2 diabetes, resulting in better control of blood sugar levels.
As a highly water-soluble substance,
Aster spathulifolius extract can be used in various forms, such as food, tablet, capsule, granular.
Product
Spathuifolius Extract Powder
Raw Materials
Spathuifolius Extract 100%
Specification
| Determination | Specification |
|---|---|
| Ingredient | Spathulifolius 100% |
| Color/Form | Brown Powder |
| Foreign Substance | Not Detected |
| Moisture (%) | Less than 10 |
| Germacrone (mg/g) | More than 11 |
| Total Plate Count | Less than 3,000 |
| Yeast & Mold | Less than 100 |
| E.Coil | Negative |
|---|---|
| Salmonella | Absence |
| Staphtlococcus aureus | Absence |
| Lead (mg/kg) | < 2 |
| Arsenic (mg/kg) | < 2 |
| Cadmium (mg/kg) | < 1 |
| Mercury (mg/kg) | < 1 |
| Storage Method | Store at room temperature to avoid hot, humid places |
|---|---|
| Shelf life | 36months (Sealed) |
| Packaging unit | 5kg |
| Packing Materials | Polyrthlene (PE) |
This information has not been evaluated by the Food and Drug Administration.
This product is not intended for diagnosing, treating, or preventing a disease.
Customer Support
+82-080-862-8200
am09:00 – pm06:00